NEUROTROPHIC KERATITIS (NK) occurs on a spectrum that ranges from altered nerve function to loss of sensation and complete epithelial breakdown. Often, patients present with initial complaints of blurred vision or mild symptoms similar to those of dry eye. Prudent diagnosis and management are vital to ensuring the disease does not progress, resulting in vision- and globe-threatening consequences.
Although NK is a rare disease, there must be frequent and time-consuming follow-up to monitor corneal healing. It is important that contact lens providers collaborate with tertiary care practitioners when comanaging later stages of the disease.
DEFINITION AND STAGING
In 2023, the Neurotrophic Keratopathy Study Group (NKSG) defined NK as “dysfunction of corneal innervation that results in dysregulation of corneal and/or cellular function. It is characterized by loss of corneal sensation and neuronal homeostasis, leading to eventual corneal epithelial breakdown and ultimately keratolysis if untreated.”1
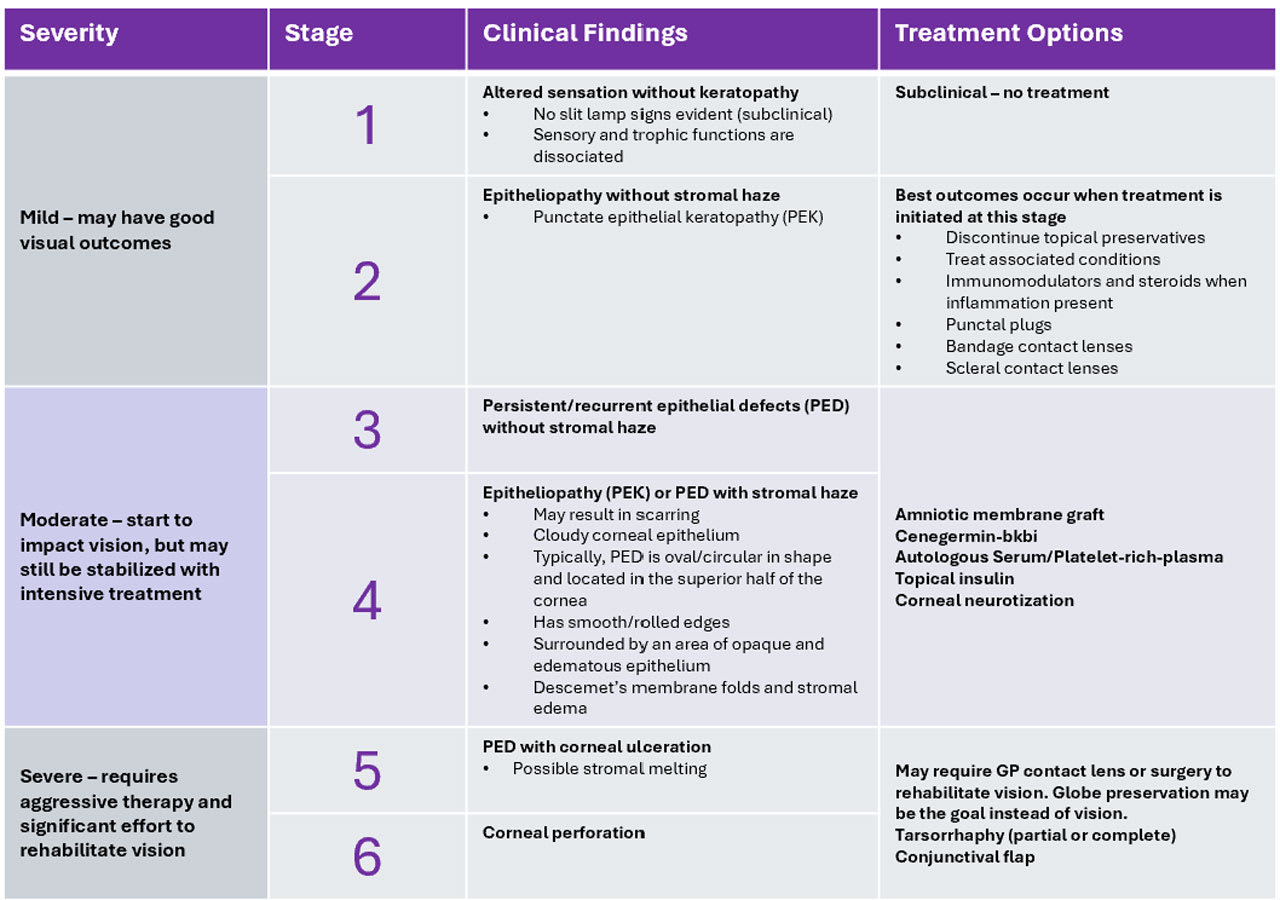
To accompany this, the study group proposed a new, six-step staging system (Table 1) to better encompass earlier disease states and provide clearer distinction between different severities to improve the ability to diagnose NK at earlier stages and determine a suitable course of treatment. This proposed six-step staging system aims to update the prior NK staging systems,2,3 which did not emphasize early stromal disease and haze/scarring as hallmarks of disease progression.
EPIDEMIOLOGY
Epidemiology on the frequency of NK is limited in the literature, but it is estimated to affect fewer than 1.6/10,000 individuals.4 Its incidence is often extrapolated from other epidemiological data on conditions associated with NK, as it affects 6.0% of herpetic keratitis cases, 12.8% of herpes zoster keratitis cases, and 2.8% of patients who have undergone surgical procedures for trigeminal neuralgia.4
Tertiary referral center data shows a frequency of 11/10,000 individuals.5 The reported frequency may increase in future studies as the newly proposed NKSG staging system increases clinician awareness of possible underdiagnosed early stages of NK.
PATHOGENESIS
Neurotrophic keratitis is a rare degenerative disease of the cornea caused by a trigeminal nerve (CN V) impairment, which leads to altered corneal sensation, loss or imbalance of corneal neurotrophic factors, and secondary alterations of the lacrimal functional unit (LFU).1,6 Disruption along the trigeminal nerve triggers the loss of corneal sensation and may occur at different levels, from the trigeminal nucleus to the corneal nerve endings.4 Both ocular and systemic diseases can cause such lesions, the most common being viral infection (herpes simplex and zoster keratoconjunctivitis), but also chemical burns, physical injuries, and corneal surgery.7
Compression of the trigeminal nerve or ganglion may also be caused by intracranial space-occupying lesions such as neuroma, meningioma, and aneurysms,8 and systemic diseases such as diabetes, multiple sclerosis, congenital syndromes (for example, familial dysautonomia and congenital corneal anesthesia), and leprosy may also decrease sensory nerve function.4,6 Regardless of the cause, impairment can lead to decreased corneal sensation, which can lead to reduced blink rate, tear secretion, and impairment of cellular metabolism and trophic factor release. These compounding factors lead to epithelial breakdown.1
There are a number of neurotrophic factors that support the normal functioning of developing and mature neurons. Nerve growth factor (NGF), epidermal growth factor (EGF), glial-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), substance P, and calcitonin gene-related peptide (CCRP) are necessary to maintain corneal homeostasis through epithelial-nerve interactions that promote epithelial cell proliferation, migration, adhesion, and differentiation.3
NGF, GDNF, and BDNF may also support the corneal epithelial stem cells at this limbus, necessary for corneal epithelialization.9 In NK, the feedback from the neurotrophic factors is impaired, leading to disrupted corneal homeostasis and poor healing.
Impaired corneal innervation also has downstream effects on the lacrimal functional unit (LFU), compromising the lacrimal glands, ocular surface, eyelids, and sensory and motor nerves that connect them. When corneal epithelial breakdown occurs, the low sensory feedback from the ocular surface causes reduced tear film and blink rate.
This creates a cycle that worsens the ocular environment because the ability to retain tears on the ocular surface is decreased, thus impairing the renewal of corneal epithelium. Epithelial breakdown then occurs due to lack of protection from the tear film and shearing stress from repetitive eyelid movements.3
CLINICAL EXAMINATION
NK is often confused with dry eye early in the disease process. Diagnosis is often made based on the clinical history of conditions associated with trigeminal impairment, presence of epitheliopathy or pigment epithelial detachment (PED), and decreased corneal sensitivity.
Mild NK (stage 2) can present with symptoms similar to dry eye, such as burning, foreign body sensation, light sensitivity, and fluctuating vision. This is why it is so critical to ensure that providers at the primary care level are testing corneal sensation as part of dry eye evaluations. With moderate and severe stages of NK (stages 3-5), redness may occur, and vision may worsen, but often symptoms decrease or disappear as the corneal hypoesthesia progresses.1
It is important to uncover the patient’s full systemic history, including any history of diabetes or other vascular accidents, brain neoplasms, corneal surgery/trauma (Figure 1a), chronic topical drugs (most commonly anesthetics, beta-blockers, and diclofenac), chemical burns, and contact lens abuse. In addition, consider a history of systemic therapies (neuroleptic, antipsychotic, or antihistamine drugs), which can cause impairment of trigeminal corneal innervation.4
Understanding of the functioning of the other cranial nerves is important for patients coming in with preexisting neurotrophic keratitis. For example, seventh and eight cranial nerve palsies may also occur with trigeminal impairment from acoustic neuroma or surgical resection of a neuroma (Figure 1b). Patients who have NK may require other testing such as blood tests for diabetes, and imaging of the brain and orbit to help with diagnosis of other neurological, orbital, or vascular pathologies.1

To ensure an NK diagnosis is not being missed, corneal sensitivity tests should be included in any anterior segment workup with corneal epitheliopathy. There are quantitative aesthesiometers, such as the Cochet-Bonnet aesthesiometer that can not only detect, but also quantify the loss of corneal sensitivity using nylon filaments of differing lengths to quantify the force necessary to elicit a blink response from each quadrant of the cornea.4
Other noncontact aesthesiometers have been developed to assess corneal mechanical, chemical, and thermal sensitivity. The Belmonte aesthesiometer stimulates the cornea with puffs of air at different temperatures, pressures, and concentrations of CO2, which enables evaluation of all three types of neuroreceptors on the ocular surface.10
Yet, in primary care, these aesthesiometers may not be readily available. A cotton tip applicator made into a pointy wisp (Figure 2) is a basic qualitative way to check for normal blinking with corneal sensation, hypoesthesia when no blink occurs but corneal sensation is reported, and full anesthesia when neither blink nor corneal sensation is reported.1

Tear film evaluation is necessary to determine whether the LFU and tear film are affected by NK. Tear volume testing, tear breakup time, tear osmolarity, interferometry, meibography, and inflammatory markers assist in evaluating the tear film stability. Assessment of the lids (to check for lid malpositioning, lagophthalmos, meibomian gland dysfunction [MGD], ocular rosacea, or other irregularities) and evaluation of the blink rate may also uncover barriers to recovery. Vital staining with fluorescein, rose bengal, or lissamine green reveals corneal and conjunctival epithelial changes in stages 2-3.
In vivo confocal microscopy can be used to evaluate corneal nerve morphology and changes in NK patients, such as decreased superficial and basal epithelial densities, reduced sub-basal nerves, and nerve length.11 Decreases in corneal epithelial and endothelial cell density and an increase in the number of hyper-reflective keratocytes was also observed.11
DIFFERENTIAL DIAGNOSIS
Mild NK (stage 2) can be mistaken for dry eye, exposure keratitis, topical drug or contact lens solution toxicity, contact lens abuse, or corneal limbal deficiency due to similar punctate corneal keratopathy and tear film abnormalities. However, the presence of corneal anesthesia and poor symptomatology are the differentiating factors. NK may be misdiagnosed as limbal stem cell deficiency due to superficial corneal vascularization and epithelial defects.
Infectious, toxic, or immune corneal ulcers, which present with ocular inflammation, stromal infiltrates, and other symptoms, are differentials for later stages of NK. The risk of superimposed infection should always be excluded by microbiological tests.4
TREATMENT
Prompt intervention based on the clinical stage of NK is critical to prevent progression of corneal damage and promote healing.1,4 Delaying treatment until stage 3-4 can lead to vision-threatening sequelae. Treat (warm compresses, lid hygiene, in-office therapies, etc.) any associated condition, such as MGD, to support corneal homeostasis by optimizing the ocular surface. With all treatments for NK, patient education encouraging adherence is key to success, as patients may not feel symptomatic enough to remember to use their medications as directed.
At stage 2, discontinue any topical ocular treatment containing preservatives. Mild cases may only require preservative-free artificial tears to improve epithelial quality and transparency and avoid epithelial breakdown. Autologous serum tears (AST) and platelet-rich plasma (PRP) have been shown to improve ocular surface health, corneal epithelial quality, and heal PEDs.14 They contain 10x greater amounts of NGF than is found in normal tears.12,13 AST and PRP may be recommended initially during early stages 2-4 or as an adjuvant to other therapies.
Ocular surface inflammation can be treated with topical immunomodulators and steroids and can be important in creating a healthy tear film.14 In later stages of NK, steroids should be used with caution due to the risk of corneal melting and perforation due to the inhibition of stromal healing.4 Punctal plugs can also be considered once inflammation is under control.
During stage 3-4 when PED is present, the treatment goals shift to avoid the development of a corneal ulcer, promote healing, and prevent recurrence of epithelial breakdown. Use of topical antibiotic eye drops to prevent infection is recommended. Amniotic membrane grafts have been shown to be effective in promoting corneal epithelial healing or reducing vascularization and ocular surface inflammation.15
Cenegermin-bkbj is a direct treatment option for moderate to severe NK (stages 3-6). It is a human recombinant NGF with a concentration approximately 1,000x that found in human tears,1 which has proven to be effective in healing >74% of severe stage NK eyes after a single eight-week course.16 Recent studies on cenegermin-bkbj in NKSG stage 2 have noted improved corneal staining and visual acuity and increased nerve density,17 giving hope that targeted intervention—even before PED development—is possible.
There is also growing evidence supporting the use of compounded topical insulin, which has been also shown to improve ulcers and PED through restoration of corneal nerves and improved epithelial cell migration.18-20 Topical thymosin B4, oral nicergoline, and combined substance P-derived peptide with insulin-like growth factor 1 are treatments on the horizon; additional studies are needed to determine their potential in treating stages 3-6 NK.
SURGICAL MANAGEMENT OPTIONS
In cases of corneal ulcers that are unresponsive to pharmaceutical and contact lens intervention, surgical alternatives are considered. Corneal neurotization is a complex surgery for stages 3-6 NK that reroutes the superior or infraorbital nerve subconjunctivally to the perilimbal conjunctival space to improve sensation and corneal innervation.1 Sensation recovery can take three to six months and nerve regeneration may take as long as six months to one year.21
In severe refractory stages 5-6, visual outcomes may be sacrificed to promote conditions for corneal healing. Partial or total tarsorrhaphy is the most simple and widely used procedure to promote corneal healing in the presence of a PED (Figure 3).4 Alternatives to tarsorrhaphy include a palpebral spring or botulinum A toxin injection of the eyelid elevator.15 Conjunctival flap is another standard surgical procedure aimed at restoring ocular surface integrity in cases of chronic corneal ulceration.22

For stromal opacification, stromal loss, or perforation, corneal transplantation (e.g., cyanoacrylate glue with bandage contact lens,patch graft, lamellar keratoplasty, or penetrating keratoplasty) may be required. Unfortunately, these options have a high risk of failure due to the lack of trophic support, leading to poor wound healing and risk of PED recurrence.23 It is important to collaborate with tertiary care providers during NK in stages 2-3 due to the risk of infection, perforation, or possible complete loss of eye vitality.
CONTACT LENSES
Two types of contact lenses can be considered when treating NK: bandage soft contact lenses (BCLs) and therapeutic scleral contact lenses (SLs).
Bandage Soft Contact LensesIt has been shown that the best use for BCLs is for stage 1 NK when lubricant drops fail to provide sufficient protection to avoid the development of a frank corneal epithelial defect.3,24 Most studies used BCLs along with other therapies (e.g., immunomodulators). Recently, BCL combined with cenegermin has been shown to successfully treat more advanced stages of NK when PED is present.25 Concomitant BCLs with oral nicergoline and topical autologous serum for severe NK at risk of perforation resulted in complete epithelialization in all studied (albeit very few) patients.26
To ensure that no opportunistic infections (e.g., microbial keratitis [MK]) occur with epitheliopathy, it is important to use prophylactic topical fourth-generation fluoroquinolones with any extended contact lens use. Figure 4 illustrates a patient who has mild stage 2 NK that worsened due to poor adherence to preservative-free artificial tears and PRP drops. A BCL and topical antibiotic were added, and signs quickly improved after one week of use.

Until new advances in contact lenses are available (perhaps in drug delivery with wear), BCLs are considered an additive therapy and not a stand-alone therapy. Preservative-free artificial tears with use are still recommended.
Scleral LensesLiterature regarding SL treatment for NK is limited to case reports and case series.27-32 The fluid layer between the cornea and lens works to provide physical protection and hydration for the cornea to preserve the epithelial integrity during blinking.
SLs have been used in NKSG stages 2-6, but best outcomes are achieved by earlier intervention with SLs, as prognosis for visual recovery is more guarded in severe stages.27, 28 SLs should be considered prior to surgical intervention to optimize cosmesis and visual function while promoting re-epithelialization.
The process of fitting the SL is no more challenging than it is for any other SL design. As with other corneal diseases, consideration for the underlying cause of the NK should be considered when fitting SLs. For example, starting with a larger diameter lens for patients with paralytic lagophthalmos,27 or careful surveillance with prophylactic treatment (e.g., oral antivirals) for patients who have herpetic NK.
Care must be taken to choose a lens material with a high Dk/t and settled lens clearance under 200µm to maximize oxygen delivery to the cornea.33 Limbal clearance is necessary to prevent damaging limbal stem cells vital to corneal epithelial vitality, and SL alignment in the landing zone is key to preventing posterior chamber debris (when too loose) or blanching and impingement (when too tight).
The challenge with SLs for NK lies not in the fit itself, but in the follow-up necessary in the ensuing days and weeks to monitor for complications and ensure that the patient is not regressing. Practitioners may consider epithelial debridement of the PED in patients in whom the edges of the defect become suspended within the posterior lens chamber with SL wear (Figure 5). This may allow for better re-epithelialization.

Several reports have shown the effectiveness of SLs in maintaining corneal integrity in cases of NK.29,30 Further, SLs have been utilized in severe stages of NK to encourage re-epithelialization of PED31,32,34 (Figure 6).

Unfortunately, the frequency of complications related to SL wear in NK is unknown. These may include corneal edema, sterile hypopyon, and corneal infections such as MK (Figure 7). By including a fourth-generation fluoroquinolone in the SL reservoir, cases of MK in patients being treated for PED decreased to zero in all study participants,32 compared to previous studies that resulted in MK occurring in 4/14 (29%) eyes treated without antibiotic use in the reservoir.31 This emphasizes the need for fourth-generation topical fluoroquinolone coverage with any contact lens use in cases of epitheliopathy.

Extended wear of SLs has been shown to create a hypoxic environment that can lead to corneal swelling twice as severe as that of physiological nocturnal expansion.35 Yet cases involving extended wear of SLs to help with re-epithelialization have been reported.34 To reduce the risk of complications (including MK), Ciralsky and colleagues proposed a standardized approach for overnight wear including daily office visits to remove, disinfect, and replace the fluid reservoir with preservative-free saline and a fourth-generation fluoroquinolone until re-epithelialization has occurred.36 Although the numbers in this study were small, no patients (n = 8) experienced MK as a complication. With extreme caution, limited use, and daily follow-up, extended wear of SLs may be an option to promote corneal healing in severe cases.
In addition to preservative-free fourth-generation fluoroquinolone use in the SL reservoir, other preservative-free alternatives may include autologous serum/PRP drops, preservative-free artificial tears, and cenegermin-bkbj to increase contact time. However, no studies have been published on these combination treatments for NK.
One practical tip worth mentioning is to caution patients during contact lens application and removal about the risk of corneal abrasion due to their reduced sensation. While patients are learning to handle SLs, BCLs can be used to temporarily protect the cornea if they are struggling to apply or remove the lenses during their training.
PROGNOSIS
NK is an extremely difficult and challenging disease to treat, even with recent advances in treatment options. The degree of corneal hypo/anesthesia impacts the prognosis and probability of disease progression, as well as the association with other ocular surface diseases.4
Early intervention in stage 2 has a good visual prognosis. Moderate stages 3-4 may start to impact vision but can be stabilized with intensive treatment. For severe stages 5-6, the prognosis for visual rehabilitation is poor, and, at times, globe preservation may be the goal instead of visual recovery.1 Patient education about the importance of follow-up care and adherence to recommended therapies is critical due to the lack of symptoms.
CONCLUSION
Early detection and intervention are of critical importance in the ongoing prognosis of NK. Patients who have NK need to be monitored indefinitely to ensure regression does not occur. Several pharmaceutical, implant, contact lens, and surgical options are available. However, caution must be taken when considering steroids or contact lens therapies due to the risk of stromal melting, sterile hypopyon formation, and infection.
Contact lenses can have a positive impact on healing and treatment in all stages of NK; however, collaboration with tertiary care providers is important for NKSG stages 3-6.
Acknowledgments: Thank you to Dr. Sophia Leung, who originally referred the severe case of NK discussed in this article for specialty lens intervention. Her comanagement philosophy is exemplary of best practices between ODs working at tertiary referral ophthalmology clinics and in specialty care areas.
REFERENCES
1. Cheung AY, Holland EJ, Lee WB, et al. The Neurotrophic Keratopathy Study Group. Neurotrophic keratopathy: An updated understanding. Ocul Surf. 2023 Oct;30:129-138.
2. Mackie IA. Neuroparalytic keratitis. In: Fraunfelder F, Roy FH, Meyer SM, eds. Current Ocular Therapy. WB Saunders: Philadelphia, PA, 1995:452-454.
3. Dua HS, Said DG, Messmer EM, et al. Neurotrophic Keratopathy. Prog Retin Eye Res. 2018 Sep;66:107-131.
4. Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014 Mar 19;8:571-579.
5. Saad S, Abdelmassih Y, Saad R, et al. Neurotrophic keratitis: Frequency, etiologies, clinical management and outcomes. Ocul Surf. 2020 Apr;18:231-236.
6. Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye. 2003 Nov;17:989-995.
7. Groos Jr. EB. Neurotrophic keratitis. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea: Clinical Diagnosis and Management. Mosby: St Louis, 1997:1340.
8. Puca A., Meglio M, Vari R, Tamburrini G, Tancredi A. Evaluation of fifth nerve dysfunction in 136 patients with middle and posterior cranial fossae. Eur Neurol. 1995;35:33-37.
9. Qi H, Chuang EY, Yoon KC, et al. Patterned expression of neurotrophic factors and receptors in human limbal and corneal regions. Mol Vis. 2007 Oct 16;13:1934-1941.
10. Belmonte C, Gallar J. Cold thermoreceptors, unexpected players in tear production and ocular dryness sensations. Invest Ophthalmol Vis Sci. 2011 Jun 1;52:3888-3892.
11. Lambiase A, Sacchetti M, Mastropasqua A, Bonini S. Corneal changes in neurosurgically induced neurotrophic keratitis. JAMA Ophthalmol. 2013 Dec;131:1547-1553.
12. Matsumoto Y, Dogru M, Goto E, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004 Jun; 111:1115-1120. 13. Park KS, Kim SS, Kim JC, et al. Serum and tear levels of nerve growth factor in diabetic retinopathy patients. Am J Ophthalmol. 2008 Mar;145:432-437.
14. Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014 May-Jun;59:263-285.
15. Khokhar S, Natung T, Sony P, Sharma N, Agarwal N, Vajpayee RB. Amniotic membrane transplantation in refractory neurotrophic corneal ulcers: a randomized, controlled clinical trial. Cornea. 2005 Aug;24:654-660.
16. Bonini S, Lambiase A, Rama P, et al; REPARO Study Group. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018 Sep;125:1332-1343.
17. Yavuz Saricay L, Bayraktutar BN, Lilley J, Mah FS, Massaro-Giordano M, Hamrah P. Efficacy of Recombinant Human Nerve Growth Factor in Stage 1 Neurotrophic Keratopathy. Ophthalmology. 2022 Dec;129:1448-1450.
18. Zagon IS, Klocek MS, Sassani JW, McLaughlin PJ. Use of topical insulin to normalize corneal epithelial healing in diabetes mellitus. Arch Ophthalmol. 2007 Aug;125:1082-1088.
19. Wang AL, Weinlander E, Metcalf BM, et al. Use of topical insulin to treat refractory neurotrophic corneal ulcers. Cornea. 2017 Nov;36:1426-1428.
20. Diaz-Valle D, Burgos-Blasco B, Gegundez-Fernandez JA, et al. Topical insulin for refractory persistent corneal epithelial defects. Eur J Ophthalmol. 2021 Sep;31:2280-2286.
21. Rathi A, Bothra N, Priyadarshini SR, et al. Neurotization of the human cornea - a comprehensive review and an interim report. Indian J Ophthalmol. 2022 Jun;70:1905-1917.
22. Gundersen T, Pearlson HR. Conjunctival flaps for corneal diseases: their usefulness and complications. Trans Am Ophthalmol Soc. 1969;67:78-95.
23. Hirst LW, Smiddy WE, Stark MJ. Corneal perforations: Changing methods of treatment, 1960-1980. Ophthalmology. 1982 Jun;89:630-634.
24. Reynolds SA, Kabat AG. Therapeutic options for the management of early neurotrophic keratopathy: A case report and review. Optometry. 2006 Oct;77:503-507.
25. Cheung AY, Shah AP, Pierson KL, Denny MR, Nordlund ML, Holland EJ. Use of Cenegermin in the Presence of Bandage Contact Lenses. Cornea. 2022 Jan 1;41:78-82.
26. Polania-Baron EJ, Graue-Hernandez EO, Ramirez-Miranda A, Navas A. Concomitant Bandage Contact Lens, Oral Nicergoline, and Topical Autologous Serum for Severe Neurotrophic Keratitis. Eye Contact Lens. 2023 Mar 1;49:116-119.
27. Weyns M, Koppen C, Tassignon MJ. Scleral contact lenses as an alternative to tarsorrhaphy for the long-term management of combined exposure and neurotrophic keratopathy. Cornea. 2013 Mar;32:359-361.
28. Witsberger E, Schornack M. Scleral Lens Use in Neurotrophic Keratopathy: A Review of Current Concepts and Practice. Eye Contact Lens. 2021 Mar 1;47:144-148.
29. Grey F, Carley F, Biswas S, Tromans C. Scleral contact lens management of bilateral exposure and neurotrophic keratopathy. Cont Lens Anterior Eye. 2012 Dec;35:288-291.
30. Alshami S, Bradley E, Nau C, Schornack M. Outcomes of scleral lens therapy in patients with neurotrophic keratopathy at a tertiary referral center. Invest Ophthalmol Vis Sci. 2018 Jul;59:1795.
31. Rosenthal P, Cotter JM, Baum J. Treatment of persistent corneal epithelial defect with extended wear of a fluid-ventilated gas-permeable scleral contact lens. Am J Ophthalmol. 2000 Jul;130:33-41.
32. Lim P, Ridges R, Jacobs DS, Rosenthal P. Treatment of persistent corneal epithelial defect with overnight wear of a prosthetic device for the ocular surface. Am J Ophthalmol. 2013 Dec;156:1095-1101.
33. Michaud L, van der Worp E, Brazeau D, Warde R, Giasson CJ. Predicting estimates of oxygen transmissibility for scleral lenses. Cont Lens Ant Eye. 2012 Dec;35:266-271.
34. Shepard DS, Razavi M, Stason WB, et al. Economic appraisal of the Boston ocular surface prosthesis. Am J Ophthalmol. 2009 Dec;148:860-868.e2.
35. Nefedov P. Do Scleral Lenses Provide Adequate Oxygen Permeability for Overnight Lens Wear? 2016 Oct 1. [Masters Thesis] Pacific University College of Optometry. Available at commons.pacificu.edu/work/sc/6dca8dd2-43f9-481d-82fc-4561358ae43f. Accessed 2024 May 29.
36. Ciralsky JB, Chapman KO, Rosenblatt MI, et al. Treatment of refractory persistent corneal epithelial defects: A standardized approach using continuous wear PROSE Therapy. Ocul Immunol Inflamm. 2015 Jun;23:219-224.